Welcome to Spot® Radio

Healthcare Packaging Podcast
with Charlie Webb CPPL
Connecting Packaging to Patients™
The voice of medical device and healthcare packaging
Join the journey of discovery in medical device packaging with our host Charlie Webb CPPL
Read more
Recent Episodes
The people behind the scenes of SPOT® Radio
MEET OUR PRODUCER
Lisa Wassberg-Webb
“I am so excited to bring to the medical device packaging community these edifying Podcast’s that will help keep us all up to date on changes in our always evolving industry. Continuing education in sterile packaging is paramount if we are to stay relevant and to meet our mission of total patient safety”
OUR DIRECTOR OF MEDIA SERVICES
Hector Garcia
Hector is the Master of Media; he has a fresh eye on industrial design born from his time in the LA design district. Hector manages all of SPOT radio’s media functions including web design and development.
Hector performs double duty as our information systems manager, keeping his keen eye on all computer functions and software systems for Van der Stähl Scientific and for SPOT Radio.
Hector also oversees all the podcast scheduling for the various podcast media platforms in order to give our listeners the best options and the best way to stream our healthcare packaging podcast “SPOT” Radio.

Visit Van der Stahl Scientific for more information.
Featured Episodes

At-home diagnostic packaging
On this episode of SPOT radio, Charlie Webb has a conversation with Jeremy Stackawitz about the future of at-home diagnostics systems. As this new market grows in healthcare it is great to ponder how packaging will support this new endpoint.
Human factor validation
On this episode of SPOT radio, Charlie Webb speaks with Carol Barnum Ph.D. from the UX firm about usability testing and human factor validation testing for medical devices and medical device packaging and speaks on the value of CX to this process.

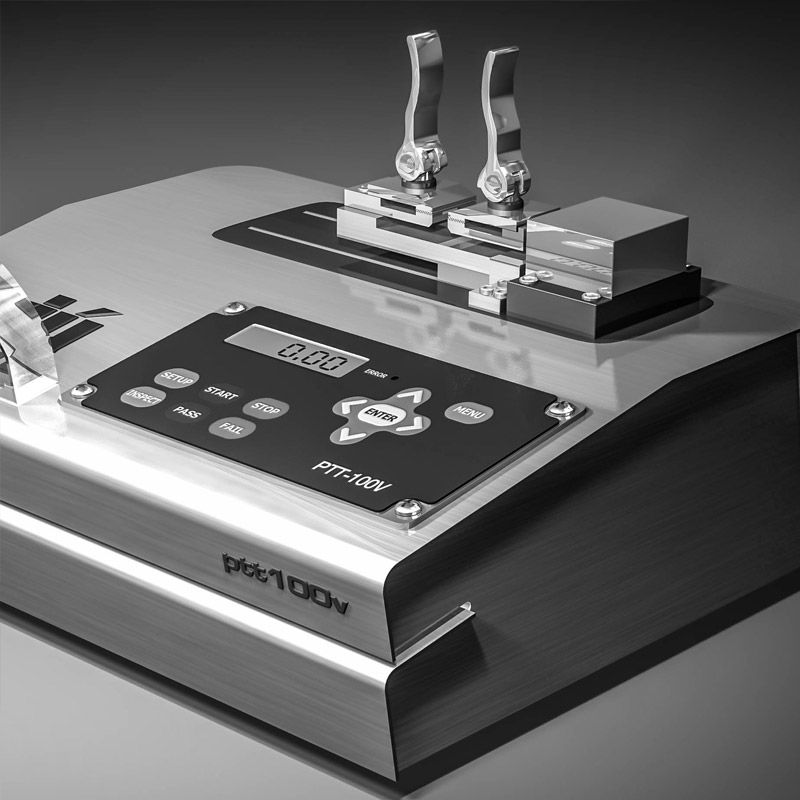
The ASTM F-88 test method
On this episode of SPOT Radio Charlie Webb speaks with Karen Greene about the ASTM F-88 medical device pouch testing method. As laboratory owners, Charlie Webb and Karen Greene provide a fresh optic to this test method and the voice of the customer.
Continuing education in medical device packaging is essential for career and company growth…
We will also discuss the latest tools to help your company stay informed on the latest in machinery, software, and materials. Our host Charlie Webb CPP speaks to the nation’s leading company’s and SME’s to help you maintain a competitive and regulatory edge in our complicated ecosystem. With 25 years in sterile device packaging Charlie Webb brings his packaging insight into discussions with our guests, Charlie says “If you are not in a continuing education mode in our demanding and risk-focused industry you will likely fall short under a siloed closed-loop packaging group, always look outside your organization to avoid the perils of incestuous data collection”



